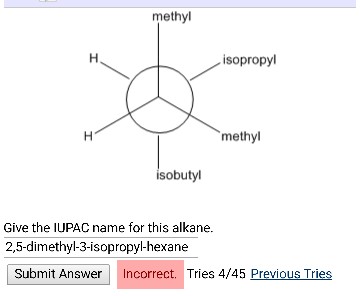
For the above isomers of hexane the IUPAC names are. Give the IUPAC name for this alkane.
QUESTION 14 Give the IUPAC name for the branched alkane pictured below a.
Give the iupac name for this alkane. Writing IUPAC name of alkane is very simple and direct method where -ane is used as suffix to indicate alkanes. We can select a suitable prefix based on the number of carbons in the parent chain and can be combine with suffix to complete the IUPAC name of alkane. For instance Here first structure is Meth aneMethane.
This causes it to have 2 methyl substituents at positions 3 4 so we would name it indicating those numbers and the prefix dimethyl which gives a proper IUPAC name of 34-dimethylheptane. C This alkane has a 5 carbon longest continuous chain length which could be numbered from left to right or right to left due to the symmetry at C-3. It has two methyl substituents.
Give the IUPAC name for this alkane. The IUPAC nomenclature is used to determines the compounds basic parent name with the help of. What is the Iupac name for alkane.
Hence C5H12 is called pentane C6H14 is called hexane C7H16 is called heptane and so forth. Straight-chain alkanes are sometimes indicated by the prefix n- for normal to distinguish them from branched-chain alkanes having the. Hence the correct IUPAC name is 36-diethyl-2-methyloctane.
What is the Iupac name of the following alkane alkane The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. The IUPAC name of alkane given is. The parent chain give the substituent of lower alphabetical order the lower number.
Prefixes such as di- tri- tetra- penta- hexa- and so on are not included in alphabetizing. Prefix the name of the corresponding straight chain alkane with cyclo-. Identify and name the substituents on the ring.
Give the IUPAC name for the following alkane. This lesson will help you understand how to use IUPAC nomenclature to name amines. Well first look at the functions of amines review alkane nomenclature and then discuss how to name primary.
Since it is an long chain paraffin or alkane so the systematic IUPAC of this compound is pentane. If the compound becomes branch compound another two isomer are possible. So total three isomeric structure is possible with molecular formula C5 H 12 and their IUPAC name are pentane 2-methylbutane 22-dimethylpropane respectively.
QUESTION 14 Give the IUPAC name for the branched alkane pictured below a. CH_3 CH_3-C-CH_3 CH_3 Common name. Neopentane IUPAC name.
IUPAC names can be generated for drawn structures in the sketcher. The name is displayed in large font above the sketcher as you doodle. Please give it a try and let us know if you encounter any issues.
A comparison table for IUPAC naming against two of our competitors is provided below. For the above isomers of hexane the IUPAC names are. B 2-methylpentane C 3-methylpentane D 22-dimethylbutane E 23-dimethylbutane.
Halogen substituents are easily accommodated using the names. Fluoro F- chloro Cl- bromo Br- and iodo I-. These are inserted alphabetically into the name in the same way as alkyl groups are included when naming complex.
Write the structure and give the IUPAC systematic name of an alkane or cycloalkane with the formulas a C8H18 that has only primary hydrogen atoms. Write the structure and give the IUPAC systematic name of an alkane or cycloalkane with the formulas a C8H18 that has only primary hydrogen atoms. How to name organic compounds using the IUPAC rules In order to name organic compounds you must first memorize a few basic names.
These names are listed within the discussion of naming alkanes. Thus putting a hyphen before the name obtained we also write the number of carbon atoms carrying the tri bond thus the IUPAC name of the given compound is obtained. Alcohol Group If an alcoholic group is present in the given organic compound then add ol at the end of the name of the corresponding alkane.
Remove e and add ol. Using the IUPAC nomenclature system name the following molecule. Convert each of the following skeletal formulas to a condensed structural formula.
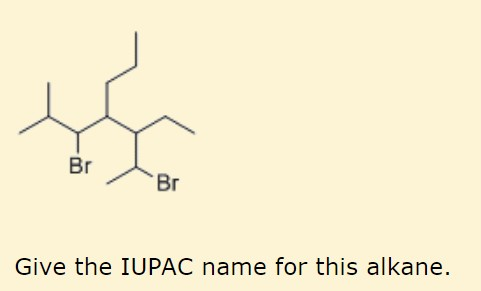
Give the IUPAC name for the following compound. Give the IUPAC name for the following alkane or cycloalkane. Give the IUPAC name for the following alkane or cycloalkane a.
Give the IUPAC name for the following alkane or cycloalkane. Black carbon white hydrogen a. To name an alkane from a Newman projection you convert the projection to a line structure.
Then you write the IUPAC name. Name the alkane shown in the following Newman projection. One C atom is at the front of the circle.
Another C atom hides behind the circle. You must trace the bonds through the structure. Pick the longest chain attached to the front C atom.
Carboxylic acids are characterised by having a carboxyl group which has the formula COOH. In a carboxyl group a carbon atom is double-bonded to an oxygen atom a carbonyl group and is also bonded to a hydroxyl alcohol group. The IUPAC suffix for a carboxylic acid is.
Simplified IUPAC rules for naming alkanes are as follows demonstrated in Example 121. Name alkanes according to the LCC of carbon atoms in the molecule rather than the total number of carbon atoms. This LCC considered the parent chain determines the base name to which we add the suffix -ane to indicate that the molecule is an alkane.